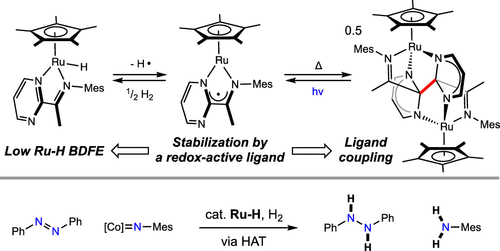
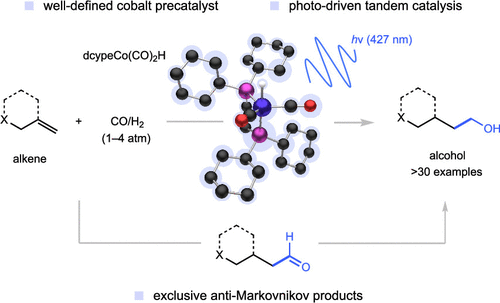
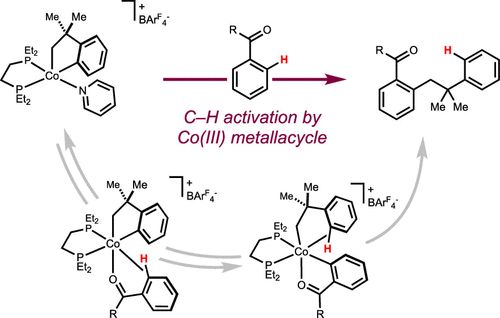
Our research focus.
Based in the Frick Chemistry Laboratory at Princeton University, the Chirik Group conducts research in two primary areas of interest: Catalysis with Earth-abundant transition metals and N2 functionalization and weak-bond formation.
Catalysis with Earth-abundant transition metals.
Catalysis is a key driver of sustainable chemistry. Most chemical industries, from commodity and fine chemicals to petroleum and pharmaceuticals, rely on catalysis as an enabling technology. Frequently, however, the transition metals we use as catalysts are among the rarest in the Earth’s crust, and difficult to extract without an environmental impact.
We are focused on catalysts based on Earth-abundant transition elements like iron, cobalt, nickel, and most recently molybdenum, which allow for potentially less expensive and more sustainable chemistry. Our primary goal is not simply to replace existing catalysts but to discover new reaction chemistry that can fully exploit the unique electronic structures of these abundant and inexpensive metals. Applications of our catalysts range from the pharmaceutical, flavor, and fragrance to commodity chemical industries.
N2 functionalization and weak-bond formation.
While the industrial Haber-Bosch process invented in the early 1900s has had a transformative impact on society – enabling mass harvests and food production for half of the world’s population and accounting for half of the nitrogen in human tissues – the fossil fuel inputs and carbon footprint associated with this reaction are significant.
Our lab seeks cleaner nitrogen fixation alternatives by developing new routes to N-H and N-C bond-forming reactions using molecular nitrogen as the building block. If successful, these methods would either circumvent the Haber-Bosch process or result in ammonia synthesis methods compatible with renewable hydrogen, inspiring new processes for the 21st century. At the core of these efforts is the formation of weak chemical bonds at or near thermodynamic potential to reduce energetic inputs and chemical waste.
What we do.
Our approach is multidisciplinary, spanning the traditional areas of organic and inorganic chemistry. At the core of our work is understanding and manipulating electron flow in first row transition metal compounds. We integrate modern spectroscopic and theoretical methods to accomplish these objectives.
Paul Chirik discusses his research, catalysis, sustainability, and applications of the Chirik Group’s work to the wider chemical industry.
Recent publications.
Sangmin Kim, Junho Kim, Hongyu Zhong, Grace B. Panetti, and Paul J. Chirik
Connor S. MacNeil, Lauren N. Mendelsohn, Tyler P. Pabst, Gabriele Hierlmeier, and Paul J. Chirik
William G. Whitehurst, Junho Kim, Stefan G. Koenig, and Paul J. Chirik

Our team.
The Chirik Group is led by Principal Investigator, Paul Chirik and Laboratory Manager, Jon Darmon. Our research team comprises a group of exceptional post-doctoral associates and fellows, graduate students, and undergraduates.